Publications

|
Varicella zoster immune globulin (VARIZIG) administration up to 10 days after varicella exposure in pregnant women, immunocompromised participants, and infants: Varicella outcomes and safety results from a large, open-label, expanded-access program Levin MJ, Duchon JM, Swamy GK, et al. Varicella zoster immune globulin (VARIZIG) administration up to 10 days after varicella exposure in pregnant women, immunocompromised participants, and infants: Varicella outcomes and safety results from a large, open-label, expanded-access program. PLoS One. 2019;14(7):e0217749. doi:10.1371/journal.pone.0217749 Despite vaccination, there were more than 100,000 annual cases of varicella in the United States in 2013–2014.1,2 Individuals at highest risk of developing severe or complicated varicella include immunocompromised people, preterm infants, and pregnant women. This publication discusses the results of an open-label, expandedaccess program that provided VARIZIG to 507 physician-identified, high-risk participants exposed to varicella. https://journals.plos.org/plosone/article/comments?id=10.1371/journal.pone.0217749 |

|
Safety and varicella outcomes in in utero-exposed newborns and preterm infants treated with varicella zoster immune globulin (VARIZIG): A subgroup analysis of an expanded-access program Duchon JM, Levin MJ, Gershon AA. Safety and varicella outcomes in in utero-exposed newborns and preterm infants treated with varicella zoster immune globulin (VARIZIG): a subgroup analysis of an expanded-access program. J Pediatric Infect Dis Soc. 2019. Infants exposed to varicella zoster virus (VZV) in utero ≤5 days before or ≤48 hours after delivery and preterm infants are at high risk for varicella complications.3-5 An expanded access program assessed varicella outcomes after administration of varicella zoster immune globulin (Human) (VARIZIG) to 43 newborns exposed to VZV in utero and 80 preterm infants exposed to VZV in a real-world setting. https://academic.oup.com/jpids/article/doi/10.1093/jpids/piz070/5644533 |
|
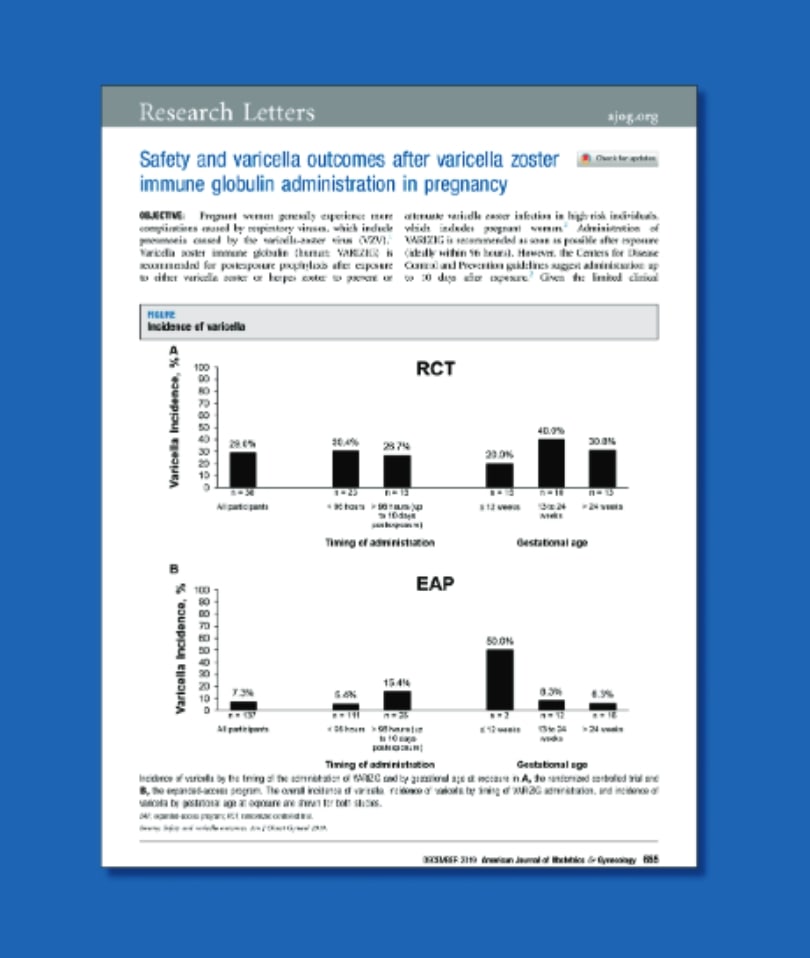
Safety and varicella outcomes after varicella zoster immune globulin administration in pregnancy Swamy GK, Dotters-Katz SK. Safety and varicella outcomes after varicella zoster immune globulin administration in pregnancy. Am J Obstet Gynecol. 2019;221(6):655-656. doi:10.1016/j.ajog.2019.07.003 Pregnant women generally experience more complications caused by respiratory viruses, which include pneumonia caused by the varicella-zoster virus (VZV).6 Given the limited clinical information with VARIZIG in pregnancy, this paper describes the safety and varicella outcomes in over 200 pregnant women who were enrolled across 2 studies of VARIZIG.7,8 https://www.ajog.org/article/S0002-9378(19)30887-7/pdf |

|
Varicella zoster immune globulin (human) (VARIZIG) in immunocompromised patients: a subgroup analysis for safety and outcomes from a large, expanded-access program Gans, H., Chemaly, R.F. Varicella zoster immune globulin (human) (VARIZIG) in immunocompromised patients: a subgroup analysis for safety and outcomes from a large, expanded-access program. BMC Infect Dis 21, 46 (2021). https://doi.org/10.1186/s12879-020-05656-6 Immunocompromised children and adults are at increased risk for severe disease and death following varicella zoster virus infection. Varicella zoster immune globulin (human) (VARIZIG) is recommended for post-exposure prophylaxis to prevent or attenuate varicella infection in high-risk individuals. https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-020-05656-6 |
References
- Centers for Disease Control and Prevention. Monitoring the impact of varicella vaccination. Updated May 16, 2018. Accessed July 15, 2020. https://www.cdc.gov/chickenpox/surveillance/monitoring-varicella
- Lopez AS, Zhang J, Marin M. Epidemiology of varicella during the 2-dose varicella vaccination program - United States, 2005-2014. MMWR Morb Mortal Wkly Rep. 2016;65(34):902-905.
- Meyers JD. Congenital varicella in term infants: risk reconsidered. J Infect Dis. 1974;129(2):215-217.
- Straus SE, Ostrove JM, Inchauspe G, et al. NIH conference. Varicella-zoster virus infections. Biology, natural history, treatment, and prevention. Ann Intern Med. 1988;108(2):221-237.
- Canadian Pediatric Society. Varicella zoster immune globulin use in neonates and infants. Can J Infect Dis. 1996;7:17-18.
- Harger JH, Ernest JM, Thurnau GR, et al. Risk factors and outcome of varicella-zoster virus pneumonia in pregnant women. J Infect Dis. 2002;185:422-7. doi:10.1086/338832
- Koren G, Money D, Boucher M, et al. Serum concentrations, efficacy, and safety of a new, intravenously administered varicella zoster immune globulin in pregnant women. J Clin Pharmacol. 2002;42:267-74. doi:10.1177/00912700222011283
- Levin MJ, Duchon JM, Swamy GK, Gershon AA. Varicella zoster immune globulin (VARIZIG) administration up to 10 days after varicella exposure in pregnant women, immunocompromised participants, and infants: varicella outcomes and safety results from a large, open-label, expanded-access program. PLoS One. 2019;14:e0217749. doi:10.1371/journal.pone.0217749
Individuals known to have severe, potentially life-threatening reactions to human globulin should not receive VARIZIG or any other immune globulin (Human). Individuals who are deficient in IgA may have the potential for developing IgA antibodies and have severe, potentially life-threatening allergic reactions. In patients who have severe thrombocytopenia or any coagulation disorder that would contraindicate intramuscular injections, only administer VARIZIG if the expected benefits outweigh the potential risks. Thrombotic events may occur following treatment with VARIZIG and other immune globulin products. Products made from human plasma may carry a risk of transmitting infectious agents, e.g. viruses and, theoretically, the Creutzfeldt-Jakob disease agent. The most serious adverse drug reactions observed in clinical trials for all subjects and patients include pyrexia, nausea, chills and vomiting. The most common adverse drug reactions observed in clinical trials for all subjects and patients were injection site pain, headache, chills, fatigue, rash and nausea. Please see Prescribing Information for full prescribing details.
To report SUSPECTED ADVERSE REACTIONS, contact Kamada Therapeutics at +972-8-9406472 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.

|
